Molecular Weight Distribution Data (latest update)
27 September 2021
When assessing the bioaccumulation potential of polymers and/or surfactants, Cefas may have reason to request molecular weight distribution data, in order to establish the proportion of a substance whose molecular weight falls below the figure of 700Da that OSPAR has agreed to use a threshold. For this purpose, Cefas has agreed with BEIS and SSM to consider 10% as the maximum proportion of material of molecular weight <700 that will fall within an assessment of “non-bioaccumulative”. This criterion will be used until such time as OSPAR is in a position to provide further advice.
Scope
Molecular weight distribution data are not required for all polymers. Cefas only requires such information if the information supplied on the HOCNF is insufficient to conclude that the substance is non-bioaccumulative, AND a bioaccumulative assessment would be expected to lead to the substance acquiring a substitution warning according to the OSPAR Pre-Screening Scheme.
Analytical Requirements
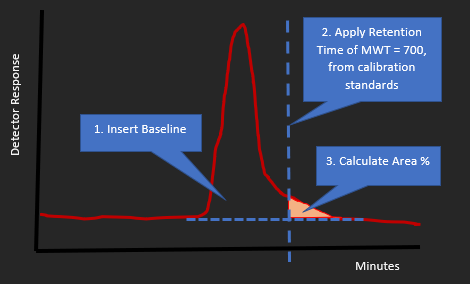
In order to provide Cefas with the required information, it will normally be necessary to undertake an analysis by Gel Permeation Chromatography (GPC). For this purpose, the OECD 119 Protocol provides basic guidance, but refers to the use of a molecular weight threshold of 1000 in defining low molecular weight content, rather than the figure of 700 employed by OSPAR. It is stressed that the calibration standards should be appropriate to cover this range.
Reporting Requirements
To ensure that adequate confidence in the analysis can be inferred from the report, it is important that Cefas is provided with:
- Full characterisation of test material(s) and calibration standards
- Full experimental details including columns, mobile phase and detection method(s)
- Chromatograms of samples, blanks and calibration standards
- An indication of the proportion of the substance whose molecular weight is less than 700, including chromatograms clearly showing how the relevant peak integration was performed
- Provide a complete profile for the component of interest, including any additional moieties that will be present in the composition of the final product. This may include solvents, by-products of manufacture, unreacted monomers, etc.
- GPC Slice table